Ref: Bussini L., et al, J Emerg Crit Care Med 2022;6:25 | https://dx.doi.org/10.21037/jeccm-22-32
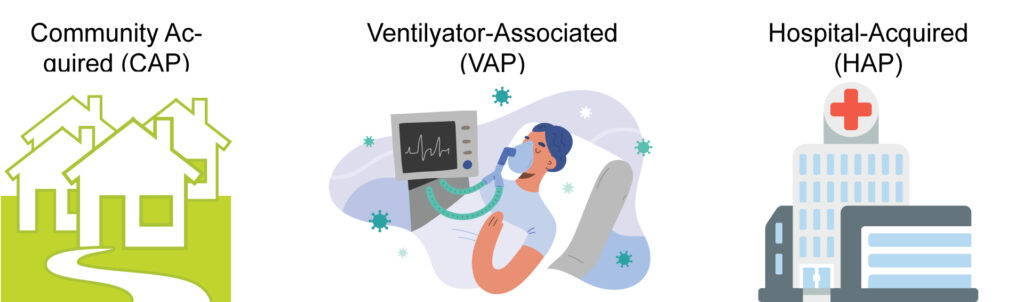
Hospital-acquired pneumonia (HAP) and ventilator associated pneumonia (VAP) remain leading causes of morbidity and mortality despite recent advances in prevention, diagnosis, and treatment. HAP is a lung infection occurring in the nosocomial setting which develops after 48 hours of hospitalization and does not appear in incubation at the hospital admission. Among nosocomial pneumonia, VAP is an infection developing in patients admitted to intensive care unit (ICU) after 48 hours of endotracheal intubation.
Indeed, the definition of VAE/VAC—that is an increase in the daily minimum positive end expiratory pressure (PEEP) of ≥3 cmH2O sustained for ≥2 days after ≥2 days of stable or decreasing daily minimum PEEP, or an increase in the fraction of inspired oxygen (FiO2) of ≥20 points sustained for ≥2 days after ≥2 days of stable or decreasing daily minimum FiO2 level—was created to frame the wide spectrum of complications related to mechanical ventilation (MV). Among them, infection-related ventilator associated complications (IVAC) are considered VAE/ VAC associated with possible pulmonary infection or nonpulmonary infection leading to respiratory deterioration (i.e., an abnormal temperature—38 ℃—and/ or white blood cell count—≤4,000 or ≥12,000 cells/mm3 —and administration of 1 or more new antibiotic for ≥4 days). Possible VAP (PVAP) refers to an IVAC with presumable lung infection supported by positive respiratory secretion or pleural fluid cultures for potentially pathogenic organisms, positive assays for respiratory viruses or Legionella, or suggestive histopathology concurrent with the IVAC.
Epidemiology
Though it is one of the most common nosocomial infections, epidemiologic data on HAP in non-ICU patients are limited and fragmented. Estimated incidence ranges from 5 to more than 20 cases per 1,000 admissions and from 2.5 to more than 6.1 cases per 1,000 non-ICU patients.
In the US, various formalized systems for ongoing national surveillance provide systematized information concerning infection rates, including pneumonia. One of the most recent large experience comes from a multicenter retrospective cohort study of 17,819 hospitalized patients from 253 US hospitals in 2012–2019 period. Among all patients enrolled, 26.5% had NVHAP, 25.6% ventilated HAP (V-HAP), and 47.9% VAP.
In Europe and incoming countries, no such reporting systems exist, and epidemiology of VAP/HAP in ICUs is inferred from national and international studies. A more recent report on pneumonia in European ICUs comes from the EU-VAP/CAP study on 2,436 patients from 27 ICUs. Among all patients enrolled, 34% developed pneumonia during ICU stay, with 18.3 VAP episodes per 1,000 ventilator-days.
The INICC is an international research network comprising centers from Latin America, Eastern Europe, Eastern Mediterranean, Southeast Asia, and Western Pacific aimed to measure and prevent nosocomial infection. The Consortium collected prospective data on nosocomial infections from 861,284 patients hospitalized in 703 ICUs in a 6-year period from January 2010 to December 2015.
The overall rate of VAP was 13.1 per 1,000 ventilator-days, higher than rates from hospitals in North America, Western Europe in the same period (0.9 per 1,000 ventilator-days). Such higher rates could be due to the extremely low nurse-to-patient staffing ratios, the hospital overcrowding, the lack of medical supplies, and an insufficient number of experienced nurses or trained healthcare workers .
Overall, prevalence of VAP has decreased in the last decades, principally as a result of implementation of prevention protocols. Main novel strategies have been priority use of high-flow nasal oxygen or non-invasive positive pressure ventilation (NIPPV) in place of intubation/ reintubation, reduced duration of sedation and MV, daily oral care, early enteral feeding, correct in-bed positioning and early mobilization.
Antimicrobial therapeutic management
Without risk factors for MDR and low mortality risk
Monotherapy covering MSSA and Pseudomonas spp (e.g., piperacillin/tazobactam, cefepime, levofloxacin, imipenem, or meropenem)
Approach to empirical therapy for HAP/VAP
With risk factors for MDR and/or high mortality risk
(I) Anti-MRSA agent (e.g., linezolid, ceftobiprole# ) + (II) Antipseudomonal agents of different classes (e.g., piperacillin/tazobactam, cefepime, ceftazidime, ceftolozane-tazobactam, fluoroquinolone, meropenem, imipenem, aminoglycoside, aztreonam) or (III) Agent with antiCRE* activity (e.g., ceftazidime-avibactam§, meropenemvaborbactam, imipenem-relebactam) or (IV) Agent with activity against Acinetobacter baumannii° (e.g., ampicillin/ sulbactam, cefiderocol)
# , not indicated in case of VAP; *, the choice of drugs with antiCRE activity should be made upon the presence of specific risk factors, rectal carriage status and taking into account the local or center-specific epidemiology (i.e., prevalence of infections caused by CRE and most common type of carbapenemase between OXA-48, KPC and MBLs); § , consider combination treatment when ceftazidime-avibactam is used in case of VAP; °, mainly based on center-specific epidemiology, previous colonization or infection. HAP, hospital-acquired pneumonia; VAP, ventilator-associated pneumonia; MDR, multi-drug resistant; MSSA, methicillin-susceptible S. aureus; MRSA, methicillinresistant S. aureus; CRE, carbapenem-resistant Enterobacterales.
Conclusions:
HAP/VAP still represents one of the most challenging complications affecting hospitalized patients. This narrative review may provide to clinicians a complete and updated summary on prevention, diagnosis and management of HAP/VAP.