Ref: William Durante, Nutrients 2019, 11, 2092; doi:10.3390/nu11092092
Cardiovascular disease is the primary cause of morbidity and mortality in the world, accounting for nearly one-third of all deaths . Although the age-adjusted mortality rate for cardiovascular disease has diminished in industrialized countries owing to life- style changes, smoking cessation, advances in biomedical research, and improvements in medical care and technologies, the aging population and burgeoning epidemic of cardiometabolic disease characterized by obesity, insulin resistance, dyslipidemia, impaired glucose tolerance, and hypertension, threatens to reverse this progress, underscoring the requirement for additional therapeutic options that target this deadly disease.
Substantial evidence indicate that amino acids play a fundamental role in the cardiovascular system. While amino acids serve as basic building blocks for protein synthesis and constitute an important energy source, a select group has been widely studied in the context of cardiovascular disease. Decades of research have established the importance of l-arginine in promoting cardiovascular health through the generation of the gas nitric oxide (NO) by the enzyme NO synthase (NOS). The release of NO by endothelial cells (ECs) regulates blood flow and blood pressure by inhibiting arterial tone. Furthermore, NO maintains blood fluidity and prevents thrombosis by limiting platelet aggregation and adhesion.
NO also protects against intimal thickening by blocking smooth muscle cell (SMC) proliferation, migration, and collagen synthesis. Moreover, NO mitigates the development of atherosclerosis by blocking the inflammatory response within the vessel wall. Interestingly, l-homoarginine, a derivative of l-arginine, also elicits beneficial effects in the circulation
l-Glutamine Metabolism
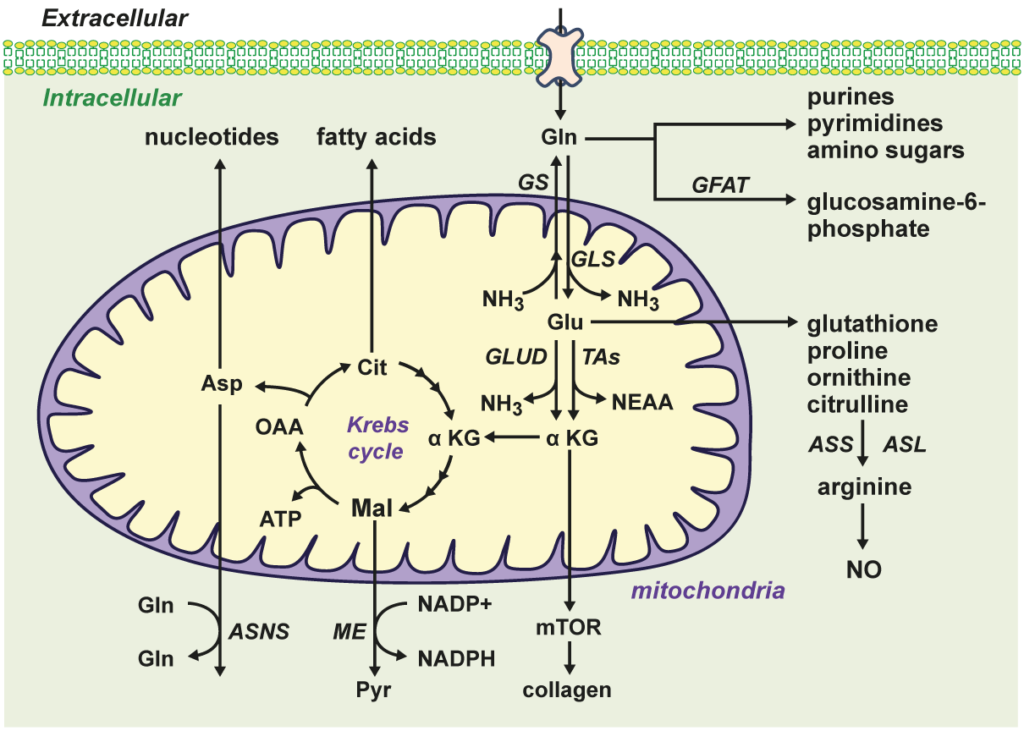
Figure-1: Overview of l-glutamine (Gln) transport and metabolism. Gln is transported into cells by various transporters and preferentially metabolized to l-glutamate (Glu) and ammonia (NH3) by the mitochondrial enzyme glutaminase (GLS). In contrast, the enzyme glutamine synthetase (GS) condenses NH3 to Glu to form Gln while the enzyme glutamine:fructose-6-phosphate amidotransferase (GFAT) transfers Gln’s amino group to fructose-6-phosphate to generate glucosamine-6-phosphate. Gln and Glu can be converted to a number of important molecules, including amino acids, fatty acids, nucleotides, adenosine triphosphate (ATP), glutathione, and Krebs cycle intermediates. Asn, asparagine; ASNS, asparagine synthetase; Asp, aspartate; ASS, argininosuccinate synthetase; ASL, argininosuccinate lyase; Cit, citrate; GLUD, glutamate dehydrogenase; _KG, _-ketoglutarate; Mal, malate; ME, malic enzyme; mTOR, mammalian target of rapamycin; NEAA, nonessential amino acids; NO, nitric oxide; OAA, oxaloacetate; Pyr, pyruvate; TAs, transaminases.
Emerging evidence indicates that l-glutamine (Gln) plays a fundamental role in cardiovascular physiology and pathology. By serving as a substrate for the synthesis of DNA, ATP, proteins, and lipids, Gln drives critical processes in vascular cells, including proliferation, migration, apoptosis, senescence, and extracellular matrix deposition. Furthermore, Gln exerts potent antioxidant and anti-inflammatory effects in the circulation by inducing the expression of hemeoxygenase-1, heat shock proteins, and glutathione. Gln also promotes cardiovascular health by serving as an l-arginine precursor to optimize nitric oxide synthesis. Importantly, Gln mitigates numerous risk factors for cardiovascular disease, such as hypertension, hyperlipidemia, glucose intolerance, obesity, and diabetes. Many studies demonstrate that Gln supplementation protects against cardiometabolic disease, ischemia-reperfusion injury, sickle cell disease, cardiac injury by inimical stimuli, and may be beneficial in patients with heart failure. However, excessive shunting of Gln to the Krebs cycle can precipitate aberrant angiogenic responses and the development of pulmonary arterial hypertension. In these instances, therapeutic targeting of the enzymes involved in glutaminolysis such as glutaminase-1, Gln synthetase, glutamate dehydrogenase, and amino acid transaminase has shown promise in preclinical models. Future translation studies employing Gln delivery approaches and/or glutaminolysis inhibitors will determine the success of targeting Gln in cardiovascular disease.
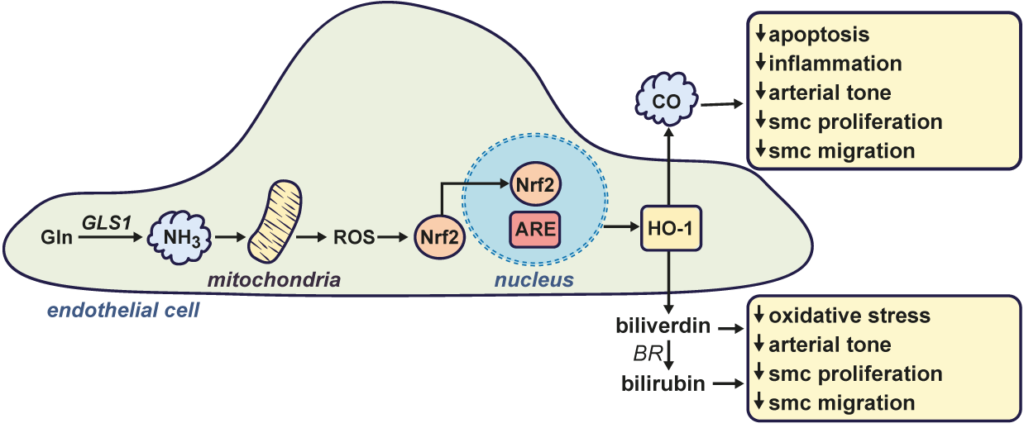
Figure-2: Role of l-glutamine (Gln)-derived ammonia (NH3) in stimulating endothelial cell heme oxygenase-1 (HO-1) gene expression and maintaining vascular homeostasis. Gln is metabolized by glutaminase-1 (GLS1) to form the gas NH3. NH3 stimulates the production of mitochondrial reactive oxygen species (ROS) which causes the activation and translocation of NF-E2-related factor-2 transcription factor (Nrf2) into the nucleus, where it binds to the antioxidant responsive element (ARE)in the promoter region of the gene to trigger HO-1 transcription. HO-1 catalyzes the conversion of heme to carbon monoxide (CO) and biliverdin, the latter being rapidly metabolized to bilirubin by biliverdin reductase (BR). CO and the bile pigments (biliverdin and bilirubin) promote vascular homeostasis by inhibiting apoptosis, oxidative stress, inflammation, arterial tone, and vascular smooth muscle cell (SMC) proliferation and migration.